Amylyx Reports the Completion of Patient Enrollment in P-III Trial (PHOENIX) of AMX0035 for Amyotrophic Lateral Sclerosis
Shots:
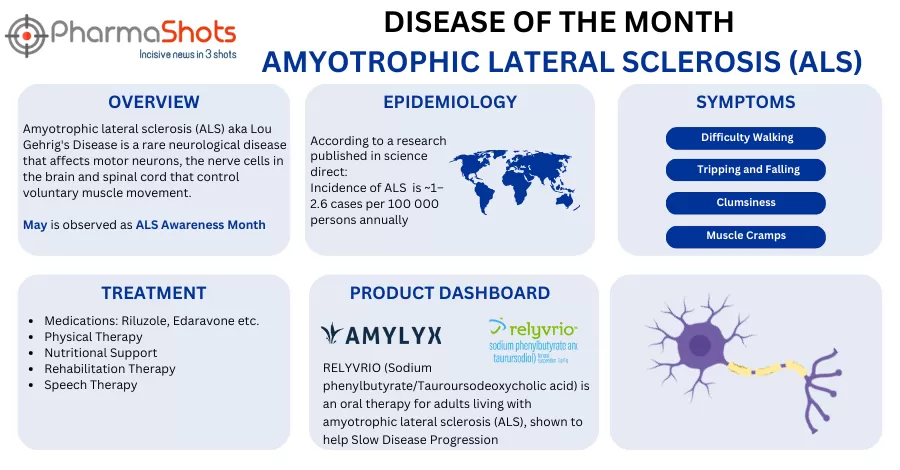
- The company has completed the patient enrolment in the P-III trial (PHOENIX) evaluating AMX0035 vs PBO in a ratio (3:2) in patients with ALS at 65+ sites across the EU & US. The trial was based on the P-II trial (CENTAUR) results
- The primary efficacy outcome will be a joint assessment of ALSFRS-R total score progression @~48wks. while the safety & tolerability will be assessed @~48wks. The results are expected in 2024
- The MAA of AMX0035 is currently under the EMA’s review for ALS with a decision expected in H1’23 & the therapy continues to advance for other neurodegenerative diseases. The (CENTAUR) trial showed a significant benefit in function & survival in a post hoc analysis, rates of AEs & discontinuations were similar b/w groups
Ref: Businesswire | Image: Amylyx
Related News:- Amylyx Pharmaceuticals Entered into an Exclusive Distribution Agreement with Neopharm for AMX0035
Click here to read the full press release

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.